The Case of Minerals and Their Silly Competitions—Who Will Win?
Minerals binding together? Need to know how to stop nutrients from blocking each other out? Got a nutrient emergency and need minerals now?
Another fertilizer article? I know, I know… But ‘tis the season and time to be putting thought into what we will use this year for our fig trees and vegetable gardens. I want a ton of tasty fruit this year and I think you do too. Plus, I hope to get you all thinking about next year too and any preparations you might need to make for that. Soon we will transition to fruit pics and what is ripening… But to get there, first we feed.
As you all may know by now, I really like to make my own gardening inputs. I like knowing exactly what is in them and exactly what I am putting on my plants that will be growing my food. I want to ensure that produce is nutritious, that it benefits my body and doesn’t cause harm with toxic residues. I also want to ensure my plants and soil are healthy with a thriving microbial life. Does it sound like I have control issues with my food? LOL, … well, maybe. However, there is a lot more to it.
All of the research shows that things need to change. We as a species cannot continue as we have with synthetic fertilizers, pesticides, herbicides, and other ‘cides because it is causing too much harm to us, our children, our animals, our planet, and to other lifeforms who call this place home. There are so many papers and articles calling out these harms that to list them all would be impossible. A simple internet search will quickly take you there.
Personally, I have lost four loved ones to cancer and have known many others with it or other diseases related to toxicities. Those losses are devastating. So, I will not be apathetic and continue trying to make well-meant but misguided past methods work for my own ease and convenience. (Is it really convenient when you are increasing pest pressure with them anyhow? Can we all say ‘Black Fig Fly’?) Maybe it is just a small piece out of a much larger broken system, I don’t know. But what I do know is that I want to use the power that is literally in my own hands to do what I can. Why hand that over to some large chemical corporation who doesn’t even know I exist outside of my dollar?
I want to feed myself food as nature intended it. I don’t want to feed myself synthetic nitrogen, phosphorus, or potassium. I don’t know about you, but I don’t think nitrogen adds that much flavor. Have you noticed how things don’t taste as good in the markets compared to how things did when you were a kid, depending on your generation, or compared to homegrown? There are a lot of reasons for that, all of which impact our own health, but one big reason is that all they were fed is synthetic NPK. The only thing that can come out is what we put in.
For produce to taste good, it needs minerals! And not just the small handful we commonly acknowledge are required, but all of them, including things or aspects we may not even know about yet. The only way to cover all bases is to use what nature itself provides because nature already knows. Nature already has it and has it in balance. No toxicities, no one mineral blocking the absorption of another mineral, no lost plant energy as it tries to convert a synthetic into a usable form— it is all in balance already in many natural inputs.
I strongly believe that one reason why terms like “organic”, “regenerative agriculture” and “natural farming” exist outside of research is because many people, whether consciously or subconsciously, innately feel that something is wrong with chemical agriculture and there is a need to get back to natural methods. I say, listen to that inner voice and use tech and modern convenience in other worthwhile ways.
I am admittedly using this opportunity to stand on my soapbox and I will step down… eventually. 😜 But in my defense, I feel it is a pretty worthy soapbox and hope to make it easier with these articles for some to join me on it.
(🎵“Will you join in our crusade? Who will be strong and stand with me?…” 🎶 Anyone get that reference? No? Oh goodness. 😅)
Let’s introduce you to another person…
There’s this guy named William Albrecht, have you heard of him? He was a soil scientist and chairman of the Department of Soils at the University of Missouri College of Agriculture. He found that soil nutrient levels affect nutrient levels in plants, farm animals, and people. Makes sense, right? Going up the food chain? Dr. Albrecht found that the minerals interrelated and affected each other and so wrote quite a few papers and articles on the subject. He focused on correcting imbalances in the soil to go with his philosophy of "feeding the soil to let the soil feed the plants."
Somewhere around the 1940s, he made a film called “The Other Side of The Fence” in which he showed animals, despite being in their own large field of grass, sticking their heads through fences to eat grass on the other side that was more nutritious. Free-ranging animals would choose the most nutritious food that was available to eat, and being better nourished themselves, would give more nutritious milk, eggs, and meat.
He also showed certain produce side by side, such as celery, lettuce, or carrots. Even though physically they both looked identical, they had vastly different nutrient contents with one having no more nutrition than a glass of water. External appearances are deceiving and do not tell us what nutrition is in the food. Sometimes the best-looking plant, the biggest and healthiest in appearance, can be the poorest in food value according to Albrecht. Nitrogen can make a plant big and green, cosmetically it looks great, but what that plant has inside is mostly nitrogen and not much else.
In a pasture whose soil has been drained of minerals, there is no good food. But on the other side of the fence, the soil is still rich. We may forget it, but animals don't. Good food grows only on good soil. — William Albrecht, Ph.D., The Other Side of The Fence.
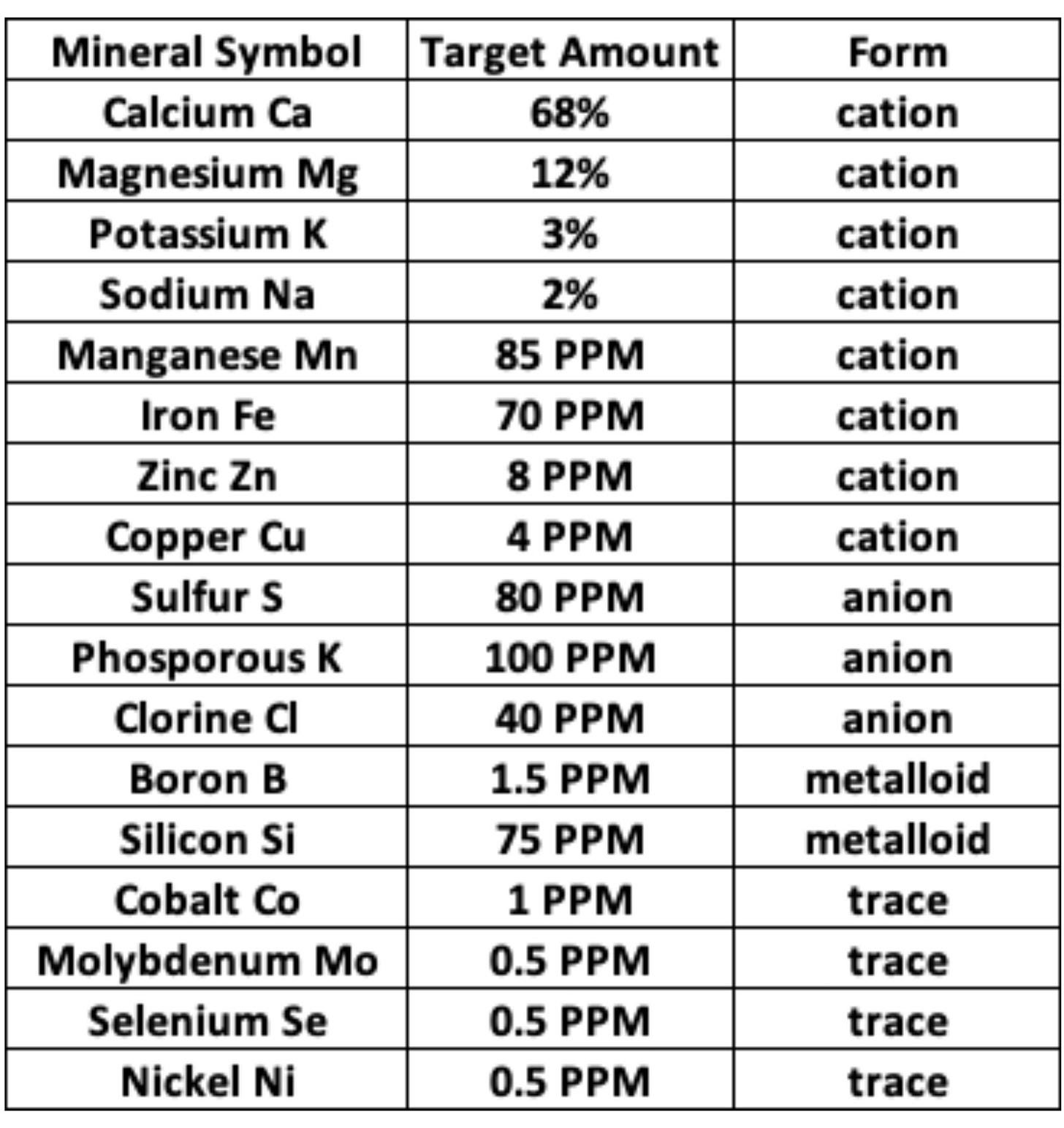
Albrecht performed tests on soils that consistently grew the highest quality crop yields and found these soils all had a similar chemistry with calcium levels being between 60-70% and magnesium 10-15%. Levels were also established for the trace elements. This is a chart that represents those “ideal” soil nutrient levels established by Albrecht and to this day is still used as a baseline for inputs:
This shows that healthy plants need at least 17 minerals, that’s a whole heck of a lot more than just NPK. Plants also want minerals to be in their ionic form. And of course, we’ve already discussed how at least 30% of these nutrients are received by plants directly through microbes. All of these minerals are what give fruits and veggies flavor and nutrient value.
Granted, I do not feel personally that this is a perfect representation of all soils that can grow nutritious food. I feel it was a limited sampling, perhaps specific to certain regions, with some assumptions thrown in there as seems par for the course. It was also primarily focused on soils with certain annual crops and not necessarily perennials or trees to my knowledge. So to me, it was a beginning of some important information. But, that said, I do feel it gives us a point of reference to aim for when supplying nutrients to plants and in balancing out soils, especially potting mixes. It helps us to see which minerals are more important.
Of course, this brings up another issue — soil tests. They too are not the most reliable because many do not show total mineral content of soils, they only measure what is currently water soluble. So a mineral can actually be present, but not reflected in soil test results because it was not in the correct form at the time the sample was taken. Other tests may use harsh acids to detect a mineral that would never be used in soil, and so may mislead on the availability of it. Though imperfect, these too can give us a point of reference for what may be missing in our land. And even if the mineral is there in a non-water-soluble form, the fact it’s not showing up on the test may indicate the microbes needed to process that mineral are not present.
The case of imbalances…
There is also the issue with nutrient blockages in plants. You can see from Albrecht’s chart that calcium is very important to plants and to fruit production. But sometimes calcium issues are not because of calcium shortages. Sometimes calcium can be present in abundance but it is not being metabolized properly by the plant leading to issues. We learned about some of the reasons for this in the article on electricity. But did you know there is a little more to it?
According to John Kempf, “unregulated absorption of potassium can lead to potassium concentrations in plant sap high enough to inhibit calcium flow into the developing fruit embryo, regardless of calcium concentrations already accumulated in the plant.” In the case of certain apple trees they found “‘Honeycrisp’ fruit contained potassium concentrations as much as two or three times that of other apple varieties grown right beside them in identical soil types with identical fertilization practices.” This same trend was observed on other crops that are sensitive to calcium issues such as chili and bell peppers. Certain varieties seem to have a disposition to hyper-accumulate potassium. This means the variety of a plant itself can have particular issues with nutrients which can cause imbalances within itself.
It is well known there is an antagonistic relationship between potassium and calcium in plant tissues. When plants contain high concentrations of potassium, foliar or soil applications of calcium are ineffective at producing the desired plant response. So sometimes it is not a calcium deficiency but a potassium excess that is affecting the plant’s ability to absorb calcium.
Even if a grower stops all applications of potassium, there can be a generous supply left in the soil from previous applications and so plants will continue to accumulate potassium. What stops this issue? Manganese. Specifically, manganese applied in its reduced form (Mn2+) will regulate potassium absorption thereby allowing calcium to be processed by the plant. “It can both up-regulate and down-regulate potassium absorption, as well as modulate potassium translocation into the fruit. When trees have a generous supply of manganese in the proper form, potassium does not move into the fruit as rapidly when the tree contains a surplus of potassium, allowing calcium to move into the fruit more readily.”
There are also issues with things like magnesium. Magnesium causes soil particles to stick together. When you have excess magnesium, the soil is too tight and oxygen is not sufficient for microbes to make the magnesium available to plants. So in the case of magnesium excess, you actually end up with deficiency. Any element that becomes excessive can cause a deficiency of the others.
Have you ever seen Mulder’s Chart before?
Mulder's Chart shows some of the interactions between plant nutrients. High levels of a particular nutrient in the soil can interfere with the availability and uptake by the plant of other nutrients. Those nutrients which interfere with one another are said to be antagonistic. For example, high nitrogen levels can reduce the availability of boron, potash, and copper; high phosphate levels can influence the uptake of iron, calcium potash, copper, and zinc; high potash (potassium) levels can reduce the availability of magnesium. So the application of high levels of nitrogen, phosphorus and potassium can induce plant deficiencies of other essential elements.
We also have stimulation effects where a large amount of one nutrient increases the need for other nutrients. For example, increased nitrogen levels create a demand for more magnesium. If more potassium is used, more manganese is required and so on.
When I look at that chart above, the amount of antagonism is insane. 🤯 Most elements are antagonistic and reduce the availability of each other. What a pain in the rear end!
Fillers! Not for wrinkles, the other kind…
There is another aspect as well that is rarely discussed. When you look at the ingredients on your fertilizer and they give you the percentages of each nutrient, do they add up to 100%? In most cases they do not. A large percentage is classified as “other”, sometimes as much as 99%. What are those “other” ingredients? They don’t tell you on the label…
In some cases, calcium sulfate (gypsum) is the “other”, sometimes it’s carbon. Some use sand, some use lime, others dolomite lime, and in the rest, who knows what in the world the “other” is? I surely don’t. But what this means is that you can think you are in balance with your ratios with what you’ve applied. But those “other” ingredients can take you right out of that balance and you have no idea this is happening. You are left mystified when you start having issues.
The solution…
Why am I talking about this? Because this emphasizes why balance is essential and simplest. I do not want to spend my time doing calculations, analyzing ratios, or whatever else to ensure I am not causing nutrient blockages. I want to enjoy my time in the garden. Whenever we apply this isolated mineral here or that isolated mineral there, we can effectively create imbalances that we do not understand or know are happening. It also shows why minerals need to be in their proper form in order to be used.
We know calcium is very important to plants. But have you ever seen a plant growing out of straight calcium powder, or straight nitrogen powder for that matter? No, isolates are not what plants want. They want soil, with an abundance of life and natural minerals. Even when one mineral is very important, they don’t want just that one.
When we look at nature, we see that minerals are returned to the soil through crop residues. Microbes break these down and they then become available to plants. Crop residues contain all of the minerals needed for that crop to grow successfully and they are present in a balanced ratio so that one nutrient does not improperly dominate. Nature does not use isolates.
A case in point— Check out the values of a fermented plant juice made from figs in ppm:

You can see that all 17 minerals are present, including the above noted manganese. Applying this as an input will not cause an imbalance, one nutrient will not block out another, and this is what nature would supply to help feed a new fig seedling, complete in its microbe-packed broken-down goo. What’s more is that in a ferment, minerals are in their ionic forms and are plant ready for absorption. No plant energy is used for conversion.
How incredibly simple to use something that is already balanced! It’s almost like a one and done. Why would I make it hard on myself by purchasing a bunch of different isolated minerals and then battle imbalances? Let’s trash the charts and keep it simple!
What if you need minerals now and not in a few months?
It is true that many natural minerals take a few months to become plant available. If you apply things like Azomite, bone meal, crushed oyster shells, or other rock dusts, etc. to your soil, plants usually cannot use them as is. The microbial life in the soil must first break these down and this is a process that takes a bit of time, which is why we typically apply them late fall through early spring before plants are actively growing.
If you can wait 2 weeks:
Another way to add in immediately usable forms of minerals is to make fermented plant extracts (FPE). This is very similar to JLF and is like a variation of it. This recipe is for a 55 gallon barrel, but you can adjust the amounts to match the size of your container. The recipe is from Mycorhizzal Planet by Michael Phillips.
Fermented Plant Extract, Mineral Rich Version
Gather approximately 20 lbs. of fresh mineral-rich weeds, herbs, grasses, and wild plants, chop roughly into 2-inch size pieces if possible, or run your lawn mower over them a few times. (Alternative: dried alfalfa meal, 1 cup per gal if using).
Place chopped biomass into a 55 gal barrel, loosely not packed firm.
Fill the barrel to 2/3rds full with unchlorinated water.
Add 2 gallons of activated Effective Microbes (EM-1).
Add rock powders* and milk at this point. (up to 5 gallons whole milk, 2 lb total of rock dust)
Fill the barrel with water until there is no gap or air remaining in it.
Cover barrel with an airtight lid.
Allow mixture to ferment for 10-14 days out of direct sunlight. (Stir every other day or so as needed to ensure the rock powder hasn’t clumped at the bottom, but try not to aerate too much.)
Open and test the pH of the liquid — it should be between 3.2 and 3.7. The plant matter should also be broken down.
If successfully fermented, roughly remove solids from the barrel with a pitchfork or handheld strainer — add solids to compost if you like. Keep the rest in the barrel and use a fine strainer as you take from it, especially if spraying it. You can also use a siphon and a strainer to separate the solids from the liquid as you fill another vessel.
Add in 1/2 lb. soluble humic/fulvic acid powder to your liquid. (If not soluble, add in above with the rock dust.)
FPE is now ready to use! Store sealed in a cool, dark place up to 90 days.
Application: FPE can be used as a foliar spray to protect plants against insects and blight and to promote plant growth, 1:500 dilution (1.5 tsp per gal) per Matt Powers. They also can be applied at a higher dilution of 3-10% per Michael Phillips. Apply every 7-14 days. This can be combined with other inputs such as seaweed extracts, neem oil, or other ingredients.
* The rock powder can be basalt, Azomite, glacial dusts, zeolite, gypsum, or limestone, etc. It depends on if you are trying to make it dominant in calcium, silica or more broad spectrum. They must be powdered minerals and not chunks.
Examples of possible combinations:
CALCIUM DOMINANT INGREDIENTS: comfrey leaf, green nettle, whole milk (up to 5 gallons), gypsum (calcium sulfate), garlic scapes (or garlic bulbs if scapes are not available), humic powders.
SILICA DOMINANT INGREDIENTS: horsetail, seeded nettle, Azomite or soft rock phosphate, granite meal and/or basalt dust, humic powders.
There is a lot of forgiveness with this recipe and the ingredients are based on what you have available locally to you. For example, I have a lot of dandelions, I do not have nettle. Dandelion is a fine substitute in this case. The basics are fresh green plant matter untreated and preferably wild, rock dusts, EM, and humic acids. Others have added in compost, worm castings, molasses, or Epsom salts to their preference.
If you have 1 week or less:
You already know about the vinegar extractions for calcium. They are quick and easy to make, contain a bunch of other minerals as well, so I highly recommend them. Seaweeds are also great for minerals. Granules provide more of a slow release, but extracts can have readily available minerals in colloidal form. The caution with seaweeds is that they also contain phytohormones, cytokinins, and other biostimulants which may or may not be desireable depending on the growth stage. KNF fermented plant juices (FPJ) can be made in as little as one week, and are a good source of minerals too.
There is another way you can get balanced minerals that are quickly available to your plants. This is through Korean Natural Farming’s diluted seawater (DSW). Seawater is naturally about 3% salinity, (ocean water varies from 3.2-4% salinity.) Seawater diluted with fresh water to between 0.3-0.1% salinity is useful for agricultural purposes. It contains most of the essential macro and microminerals that are important for healthy plant growth (Turekian 1968, DOE 1997, Motavalli and Marler 1998).
Take a look at some of these essential plant nutrients in seawater and the plant benefits:
You may not know this but the use of seawater for irrigation in agriculture has been ongoing for decades (Mount and Schuppan 1978, Feign 1985, Glenn et al. 1998, Sgherri et al. 2008). Sea salt has also been studied as a source of sea mineral solids for foliar and soil treatments (Heckman and Orton 2010).
Seawater combined with other inputs stimulates the growth of beneficial microorganisms that can help to suppress disease. It has been used with other KNF inputs (Park and DuPonte 2008) and as a soil treatment drench prior to planting fields. It is also used as foliar spray during the reproductive stages or fruiting period of a plant’s life cycle (Cho 2010), applied on a set schedule once every three weeks. On fruiting trees DSW is used as a light spray two weeks prior to ripening to increase sweetness or brix within fruit.
The mineral balance of seawater is the same as the amniotic fluid of a mother, the blood plasma of humans, and the body fluid of plants. Seawater acts to chemically flocculate - become fluffy- the soil adding airspace, water holding capacity, and homes for microbes. You can add diluted seawater to exhausted land, for building soil texture and to relieve compaction.
How to do it:
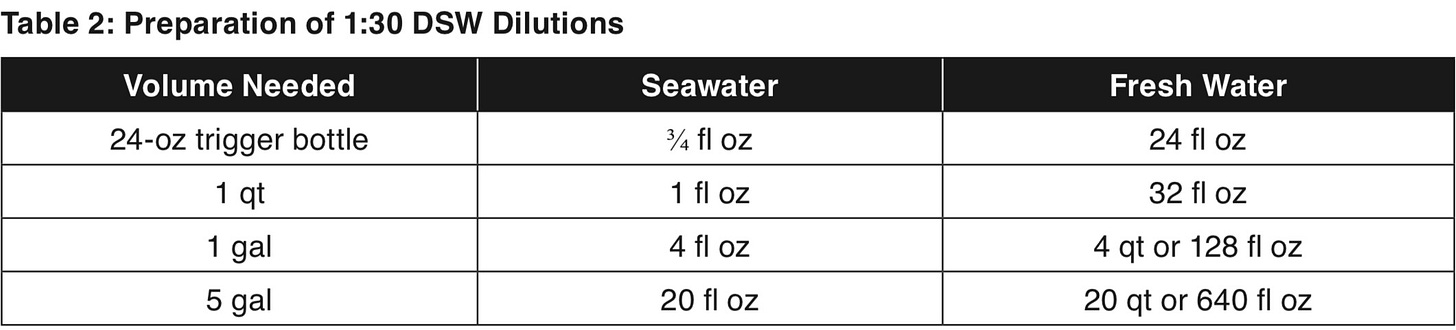
To make 0.3% salinity, dilute seawater 1:10 with fresh water
To make 0.1% salinity, dilute seawater 1:30 with fresh water
Alternatively with natural sea salt (make sure this is sea salt with high mineral content and lower salinity):
To make 0.3% salinity, add 3g sea salt for every liter of fresh water
To make 0.1% salinity, add 1g sea salt for every liter of fresh water
Make sure the salt has fully dissolved before using.
If collecting seawater:
Gather the top inch or two of water from the sea near where the sea crashes on rocks and is foamy or near the shoreline. Some brackish water is okay, but avoid places where large quantities of freshwater is entering the sea.
Pour the collected water into a large bowl and allow it to sit, uncovered, for 24 hours. This allows evaporation, aeration, concentration of solids, and the inoculation of airborne microorganisms to occur.
Keep seawater in a clean glass jar with a lid out of direct sunlight. DSW does not have a long shelf life and should be used soon after preparation. If DSW takes on a foul odor or if a white haze or white mold forms in the jar, discard it and make a new batch.
To enhance ripening, one source recommends:
2 weeks before harvest apply 1:20
1 week before harvest apply 1:15
2 days before harvest apply 1:10
For soil treatment:
Drench 1:30 or can go 1:15 if lots of rainfall.
Application:
DSW is typically always applied at the 1:30 dilution, or 0.1 salinity dilution if using sea salt. It is always best to start at a higher dilution to test the effects on your plants before going to a lesser dilution. You can mix the dilution with other natural inputs as well. Apply DSW onto plants with a watering can, sprayer, or irrigation system to encourage ripening, ideally early in the morning or in the evening. For soil treatments, moisten the soil prior to applying DSW, then lightly water again after DSW application. DSW can be applied monthly or as little as once a year. It can be applied at 1:30 dilution every three weeks during fruiting stages.
Always make sure that the dilution (1:30) of DSW is made properly. Because there is a certain amount of salinity, you do not want to overuse this. If plants start to show signs of yellowing a few days after spraying with DSW, lightly water plants and broaden DSW dilution for the next applications. (If the problem persists, it is possible that there is something wrong with the seawater used, such as the cation-exchange capacity, the salt content, or unnatural contaminants that may have been in the seawater.)
Look at all of the minerals in seawater, this is why it has benefit for plants:
Is nature backwards or so advanced it’s beyond our ability to recognize it? [*Cough, cough…Paging Dunning-Kruger…cough*]
Natural methods at some point were given kind of a backwards implication and the progressive, advanced approach was aimed at chemicals and lab created inputs. But I don’t know… When did mankind ever make a moon, a sun, stars, or a whole entire planet with a vast diversity of life that cycles on its own without anyone having to do anything? Seems like nature is way more advanced than us and our little laboratories, no? Especially so since it made us and we merely take from it and imitate it with our inventions. Perhaps a few steps backward is the forward step for our time? Let’s keep up with what we are finding out about soil & plant health instead of holding on to something that needs to end or at the least be limited in its application. Let’s use what is proving to be superior. Upwards and onwards, my friends!
A blast from the past for you! A copy of The Other Side Of The Fence by William Albrecht for your entertainment.
If it was true then, imagine how much more so it is today, since things did not change as Dr. Albrecht had hoped…











A lot of great information and insights here.Mineral chelates made from plant extracts or fermented plant teas…are really valuable tool to stave off mineral deficiencies. Macro and micro nutrients can be pricey. This recipe you show is great and approachable.