Ever Wish You Could Have A Force Field For Your Plants? —Part 2: Electricity
Did you know plants have an internal bug “zapper”? Let’s talk about the amazing power plant!
This is the second article in a three-part series exploring how to prevent and deal with insects and disease. We have more power in our hands,(hey, hey see what I did there 😏,) than we think. We are not helpless and our plants are not helpless. Read on to see how to get the power, my friends…
“Look! It's moving. It's alive. It's alive... It's alive, it's moving, it's alive, it's alive, it's alive, it's alive, IT'S ALIVE!”
…Okay, not really quite like that. 😏 But still… Did you know life is electric? There are electric fields all around us. There is electricity in our atmosphere. Our hearts and brains have electromagnetic fields. The earth resonates at 7.83 Hz. Bees, butterflies, and birds navigate through sensing electromagnetic fields. It is amazing and, dare I say, electrifying!
Electricity is in us too. Did you know that if you have a multimeter that measures voltage, resistance, and current, you can put the ground lead into the soil and you can put the other end on your tongue while standing barefoot and you will measure a voltage and resistance from your tongue to the soil? And did you know that after wearing shoes all day, you build up ions in your body from not being grounded? When you take off your shoes and stand on the ground, you actually discharge that buildup into the earth. There are researchers who have studied the electrical resistence of certain farm animals as a measurement of health, each type of animal had their own specific resistance number. All living things have a flow of current going into the soil.
So do plants.
“What drives life is a little electric current, kept up by the sunshine” — Albert Szent-Gyorgyi
A plant is not just a plant, it is literally a power plant! Flowers are antennas constantly giving off electromagnetic waves to let the world know what it has to offer. The leaves are solar panels collecting energy from the sun and moving that energy through the plant, down to the roots and into the soil. The sap flow is really an electric wire with a current, it moves charged mineral ions giving power to its parts and to microorganisms. All of that energy flows into the soil which is a battery of organic matter storing electrical charge.
An•ten•na (ăn‑tĕn′ə): An apparatus for sending or receiving electromagnetic waves.
Do you recall that insects have antennas? Those antennas are moving all of the time, they are never still. When the antennas are spread really wide apart, they pick up a broad range of electromagnetic frequencies. The antennae come closer together when they find a frequency they want to zero in on which helps them to know which direction to go in to find the location of that specific frequency.
If you watch a bee getting ready to leave the hive, the first thing it does is to use its front feet to wipe its antennas to make sure nothing is there to impede signals and so it can actively sense the world around it as it is flying through the air.
As bees are flying, they build up a positive static charge. The pollen on a flower has a negative charge. Because of this difference in charge, the pollen pops from the flower onto the bee. Once the negatively charged pollen has been removed, the charge of the flower changes. And so the next bee that comes along senses this with its antennas and knows not to bother going to that particular flower because it no longer has pollen.
These antennas are everywhere in a variety of shapes and sizes. We see them in plant hairs, or trichomes, as well. They are all over the surface of a plant and they allow a plant to not only sense electromagnetic radiation, but also to absorb electromagnetic radiation to perform functions within those trichomes. Consider the hair on the back of our necks and on our bodies, which is attuned and reactive to our environment. These antennas are all about environmental perception of the electrical fields around us.
So what is an insect eating your plant seeing? Insects eat unhealthy, or oxidized, plants. An oxidized plant emits infrared radiation indicating that it is of a particular type that is useful to a specific insect. The fact that an insect starts eating your plant is because the plant in its unhealthy form is digestible to that insect, or suitable for its eggs and larvae, and is advertising itself as such to it. This is nothing more than nature doing what it needs to do to keep things clean and cycled.
Plants have specific voltages and acidities within their different parts. There is a gradient within each plant from top to bottom and from leaf tip to leaf base. Even the xylem and phloem flow have different voltages and acidities. It is this which insects and pathogens are attracted to when they are in the correct range for them. By increasing the health of the plant, the electromagnetic waves from that plant are going to change letting the insects know a plant is not digestible to that particular insect. This is why you can determine the health of your plant by the insects eating it.
(For example, aphids eat plants that are too alkaline and nematodes are in acidic, oxidized soil with low organic matter.)
It’s all about the protons and electrons
Olivier Husson, an agronomist, has put in extensive research for many years studying the acidities (pH) and voltages (Eh) that different insects, fungi, bacteria, and plants need to live. Technically, pH measures protons. Something becomes acidic when it gains protons and it becomes alkaline when it loses protons. Eh measures electrons which is expressed by voltage. When something becomes oxidized, it has lost electrons. To keep this simple, we will refer to Eh as “voltage” since it is measured in millivolts (mV) and we will refer to pH as “acidity” since that is how most relate to it.
All life requires specific voltage (electron) and acidity (proton) conditions to survive. When bacteria, fungi, and insects occur on a plant, it is because the plant voltage and acidity has moved up into the range where these things function. The fungi, bacteria and insects themselves can also affect the local voltage and acidity.
Voltage and acidity have a lot to do with plant defense. We can make plants unfavorable for pests and disease through modifying voltage and acidity conditions in soil and plants. The idea is to prevent the pest or disease instead of waiting for it to happen and then trying to kill it.

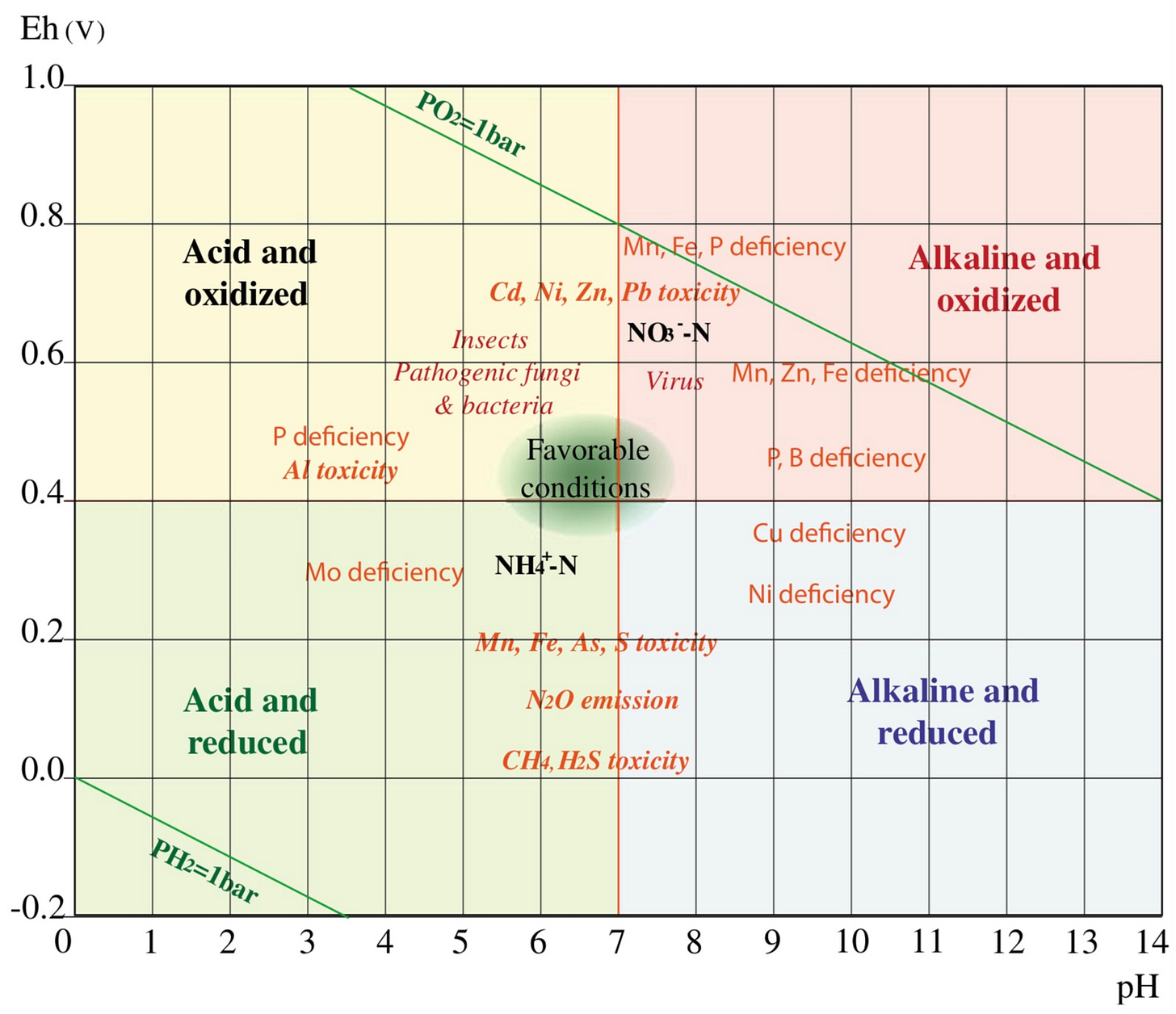
The graph above shows us the voltage and acidity of healthy plants and unhealthy plants, this affects the frequencies they emit. It shows at which voltage and acidities insects and different pathogens thrive. It also shows oxidated conditions in the soil at the top and anaerobic, compacted soil conditions at the bottom. This graph gives a lot of information and explanation for why problems happen when, so I encourage taking a few minutes to examine it.
You can see that we want our plants in the “Healthy Plants” zone. Moving a plant’s voltage and acidity up and to the right puts it in the unhealthy zone where things are oxidized and stressed, and if we go too far down to the bottom left we are in anaerobic and acidic conditions, which is also not good.
Plants cannot move, as we know, they are stuck in one spot. That being so, they are subjected to all sorts of natural impacts, such as drought, excessive rains, cold and hot temperatures. They have to deal with biological insults of pests and lack of food or nutrients. When this happens to humans, we do something about it. If we get too hot, we move to a cooler spot; if it’s cold, we get a blanket or stand by a heater; if we are hungry or thirsty, we eat or drink; if a bug bothers us, we swat it. Plants can’t do that, yet they have survived down through millennia quite successfully. The way they survive is by adjusting circumstances within themselves and in the soil. They literally change the electrical system.
When a pest is bothering a plant, it will quickly do an oxidative burst to move the electrical field past the range of tolerance for that insect or disease. It then will quickly correct by releasing salicylic acid to bring itself back down to the level it prefers. There is your bug zapper, but a plant needs to have enough energy available to be able to do that.
Plants, like all living organisms, need to sustain homeostasis. This is maintaining an internal equilibrium by adjusting physiological processes. And so whenever something is happening to take them out of that equilibrium, they expend energy to adjust circumstances to bring things back to balance.
Whenever we do something to a plant, whether that is watering it, putting it in the shade, changing its soil, or adding in nutrients, etc., it is going to have an effect on a plant’s voltage and acidity that is going to require the plant to adjust in order to keep balanced. This is going to take energy from the plant each and every time.
That isn’t to say you shouldn’t do the necessary things. And this isn’t about getting expensive sap tests and analyses to know exact deficiencies or walking around with our meters checking the voltage and acidities of everything. (Though you certainly can do that if you wish.) These parameters fluctuate a lot, just even depending on the specific part you are measuring, the time of day, if you recently watered, etc. It can be difficult to get an accurate reading for the average backyard grower’s needs since we are not in controlled settings. Thankfully, Olivier Husson has already done the work and has mapped everything out which makes it very easy for us.
The graph above is a tool to understand how much adjustment we are requiring of a plant when we intervene in some way. It shows us the amount of expended energy that will be needed to return to balance. Some things have a huge impact, and some things have minor impact. This is important because a plant can use its energy to grow new leaves, to grow quality fruit, to initiate its defenses when needed or it can spend energy trying to maintain equilibrium from all that we are doing to it. We need to minimize our impacts so that we don’t require energy expenditure that is unnecessary. And if we do have certain insects or diseases affecting our plants, then we need to make corrections to get them into the right voltage and acidity. This is all about managing energy so that plants stay out of the insect zone.
We can use Husson’s graph to predict the outcome of our interventions. We can see if our actions will be detrimental to the plant or help the plant. It is up to us to facilitate this natural process. Let’s see how to use the graph.
Energy impacts
Oxidation — Raising Voltage
Too much drainage- drought
Fire
High temperatures
Chemical fertilizers (everything that ends in ‘ate or contains chlo’; except urea.)
Pesticides, they block electron transfer
Herbicides
Fungicides
Insecticides
Plowing or tilling
Bare soil, no plants
Low organic matter on soil
It’s really all about oxidation and the loss of electrons, this is what takes us up into the spectrum insects and pathogens like, which is very easy to do.
For example, when we till the soil, we are exposing the soil to oxygen. In natural soil, the top is low voltage and when you go down you have higher voltage. When you plow, you reverse this. That oxidizes the soil which moves conditions up and to the right on the graph. If you have a healthy plant, everything is going well, and then you till the soil around it, you have changed the conditions right into the area where pathogens and insect pressure will take hold. So we can say, if I am going to till, I know I will move things towards oxidation and this has the potential to make conditions conducive to pest and insect pressure. This tells us to make a correction.
Even water can have a huge effect on voltage. If it is raining and everything is becoming saturated, voltage will drop very fast, and with drying it goes up very fast. You can have differences as much as 600 mV between wet and dry. Really the amount of saturation only matters if the condition is being maintained, think dry desert versus wet rice paddies. In drought we have oxidized conditions, taking things up on the chart. In waterlogged situations, we have anaerobic conditions taking things down to the bottom. And when soil is alkaline as well, this favors so-called pathogenic bacteria.
Whenever we apply chemical fertilizers or any ‘cide, these are your pesticides, herbicides, fungicides, insecticides, etc., these cause oxidation and move things up and to the right into pest and disease zones. Pesticides and fungicides block electron transfer so that it can fight a pest or fungus with over-oxidation. In certain situations, Husson found that even after three weeks from applying an herbicide there were differences of 15-20mV which is a big difference that can easily take you from the healthy zone to an oxidized state and keep you there for a length of time. (Hello, insects! 🪲) With chemical fertilizers, most minerals are in oxidized forms. Everything that ends in ‘ate or contains chlo’ will cause oxidation.
When things are too oxidized, the plant needs to correct and cannot develop its capacity of photosynthesis and its capacity for reduction. The energy is mainly going to correction. This is the red-light district for insects, plants are blasting away the infrared energy waves letting the insects know they do not have the energy reserves to fight them.
Reduction — Lowering Voltage
Lowering oxidation is harder than raising but can be done. These are the things that reduce oxidation:
Sun on the plant for photosynthesis
Water irrigation
Soil structure — fundamental to keep the water and humidity
Mulch
Organic matter
Biological activity
Urea (for the chemical inputs)
Paramagnetism, this is a newer possibility for reduction, using materials with paramagnetic qualities (basalt, granite, some types of biochar, etc.)
Boosting photosynthesis reduces oxidation and is a preventative against pests. The more photosynthesis that takes place, the more energy a plant has. This energy is then used to grow more leaves, which then increases photosynthesis even more, increasing available energy even more. This is also related to organic matter on top of the soil. The more leaves a plant has, the more biomass it can return to the soil at the end of the season to build up the organic matter. Photosynthesis is also one way how the soil gets energy, through a plant’s photosynthate.
Soil structure and organic matter define the holding capacity of the system. The less organic matter you have, the more oxidized the soil is. With low organic matter and low biological activity, the plant needs to pump a lot of its photosynthate into the soil to reduce oxidation around the roots and to feed microflora to build them up. All of this energy that is going into the root area to control voltage and acidity is not available for making new leaves. The leaves are what allow for photosynthesis but instead, the plant is spending most of its energy feeding microbes. Organic matter, mulch, and soil structure are essential for regulating water and biology. Without it, a plant is trying to attract and feed something that just isn’t able to be there.

The above graph shows the change in voltage as soil becomes wetter in chemically maintained soils versus biological soils. If we have soil with low biological activity, the voltage can swing by over 300mV very quickly by going from a dry condition to a wet condition. This takes us straight into the range of pathogen and insect pressure. But in soil with high biological activity, we do not get that huge swing. Note how it stays at a fairly even level as the saturation increases. This shows how important biological activity and organic matter are in maintaining the voltage of the soil when it rains or we over water. It buffers the voltage and acidity effects of water.
Plants are able to considerably alter voltage and acidity with root exudates, these are the products of photosynthesis. They use these exudates to attract the biology they need to further alter their environment. As a group, microorganisms have a high ability to modify the environment. They lower voltage because they consume oxygen, they are also able to neutralize or acidify pH. So whenever we are increasing biological activity, we are moving things down and to the left.
Fermentations and extracts are low voltage and low pH and they add microbiology to the soil. They help to move the soil and plant into the right direction. Applications of microorganisms (possibly EM 1) on tomato plants had a reduction of 30mV 2 hours after application. So if you see a potato beetle eating on your potato plant, you can foliar spray a vinegar extract onto your plant to move the plant almost immediately from the insect-attracting spectrum to a lower spectrum which repels them.
(How much of an effect this has depends on how poor or great the health of the plant is to begin with— how much movement on the spectrum do we have to do?)
Charging the battery
At 400mV, the soil battery is fully charged. The carbon and microbiology are the charge level of the battery. The more you have, the more your battery is charged. When you raise the voltage, going towards oxidation and loss of organic matter, you are discharging your battery. When you are very low voltage, full of organic matter and water, it is too much energy. It’s like a carburetor of an engine flooding with fuel but no room for air, so you can’t use the energy. Or compare it to a sand dune versus a peat bog. Both are extremes and we need the middle. In other words, we don’t want 700mV and we don’t want 0mV, we want 400mV, 6.5pH and we want to maintain it there to the degree possible.
To have a charged battery, we need nutrients. Many are familiar with the pH chart for mineral availability. About 7 pH is where plants have access to most of the minerals according to the chart.
But when I said electricity affects everything around us, I meant it— it affects mineral availability as well. We know that characteristics of soil are not just acidity but also include voltage. Plants prefer minerals in their ionic charged forms. So we need to rethink mineral availability in line with voltage.
Have you heard of Pourbaix diagrams? They have been around since the 1950s and are used to determine the phase stability of minerals. We need to understand what phase certain minerals are in at different voltages and acidities to determine if they are available to plants.
Look at the above pH chart for manganese. It says at that ideal soil pH of 6.5, we can have all the manganese we want. That’s one part of the picture, now let’s bring in voltage. This is a Pourbaix diagram of manganese:
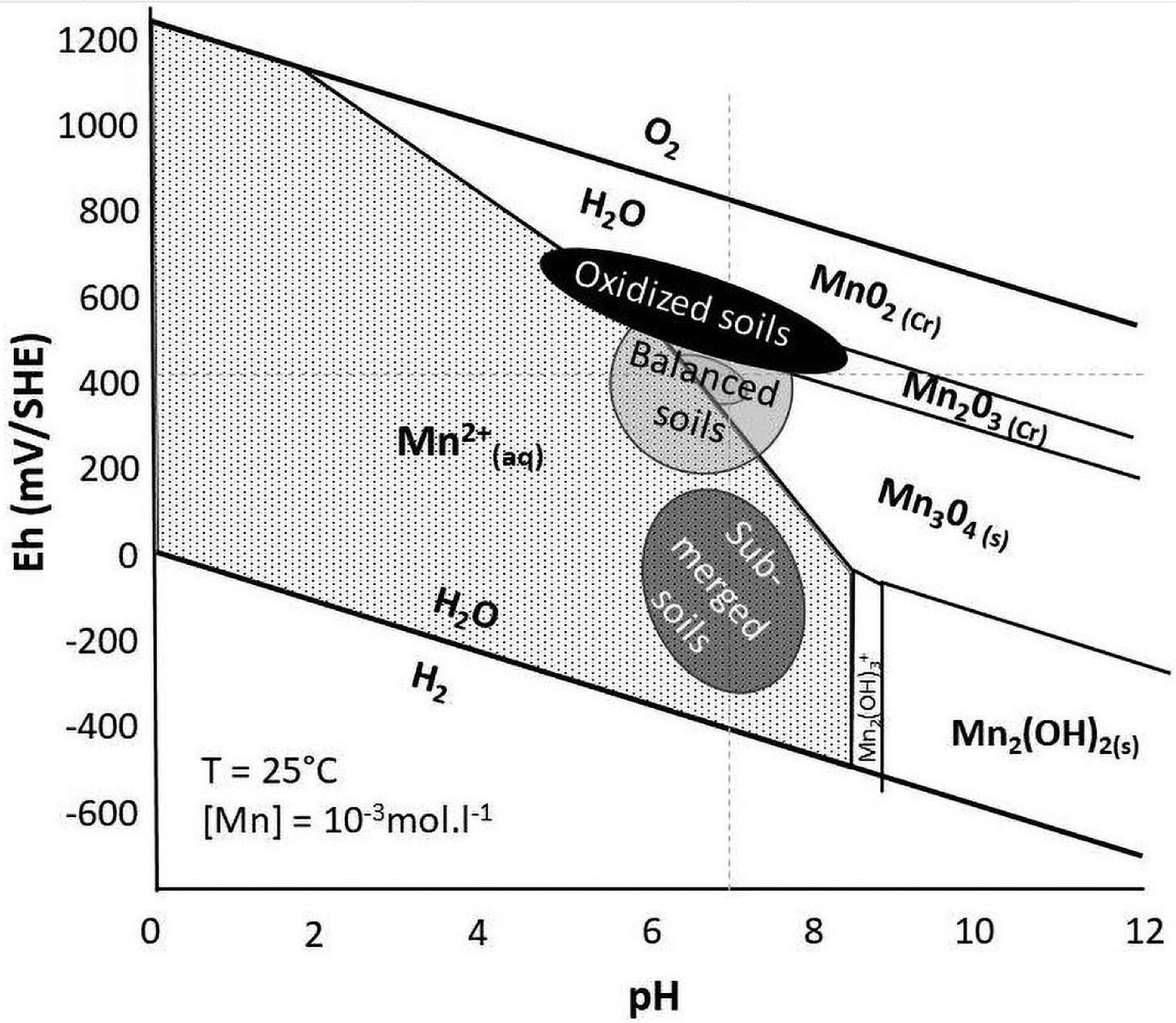
The type of manganese that is absorbable by plants is the aqueous form Mn2+, the positive ionic form. Looking at the chart with the voltage (Eh), we see that at 6.5 pH, if we have a voltage of 600-700, then that manganese is not available. You can have all of the manganese in the world in your soil, but if the voltage is wrong, your plant isn’t getting any in that form and it is going to have to expend a lot of energy to convert that oxidized manganese into the aqueous form. So the pH chart is misleading in not showing the voltage characteristic that is involved as well. It is all about the energy for everything.
In fact, if you have a deficiency in manganese and then you apply manganese to the soil in an oxidized form, you can actually make the deficiency worse for a short time. This is because that oxidized form has moved soil voltage and acidity up to a more oxidized state. It has not helped the equilibrium but has made it worse. The only way to get it in balance and to correct the deficiency is to do a foliar application of the plant available form, Mn2+ (aq).
This is a Pourbaix diagram of iron:
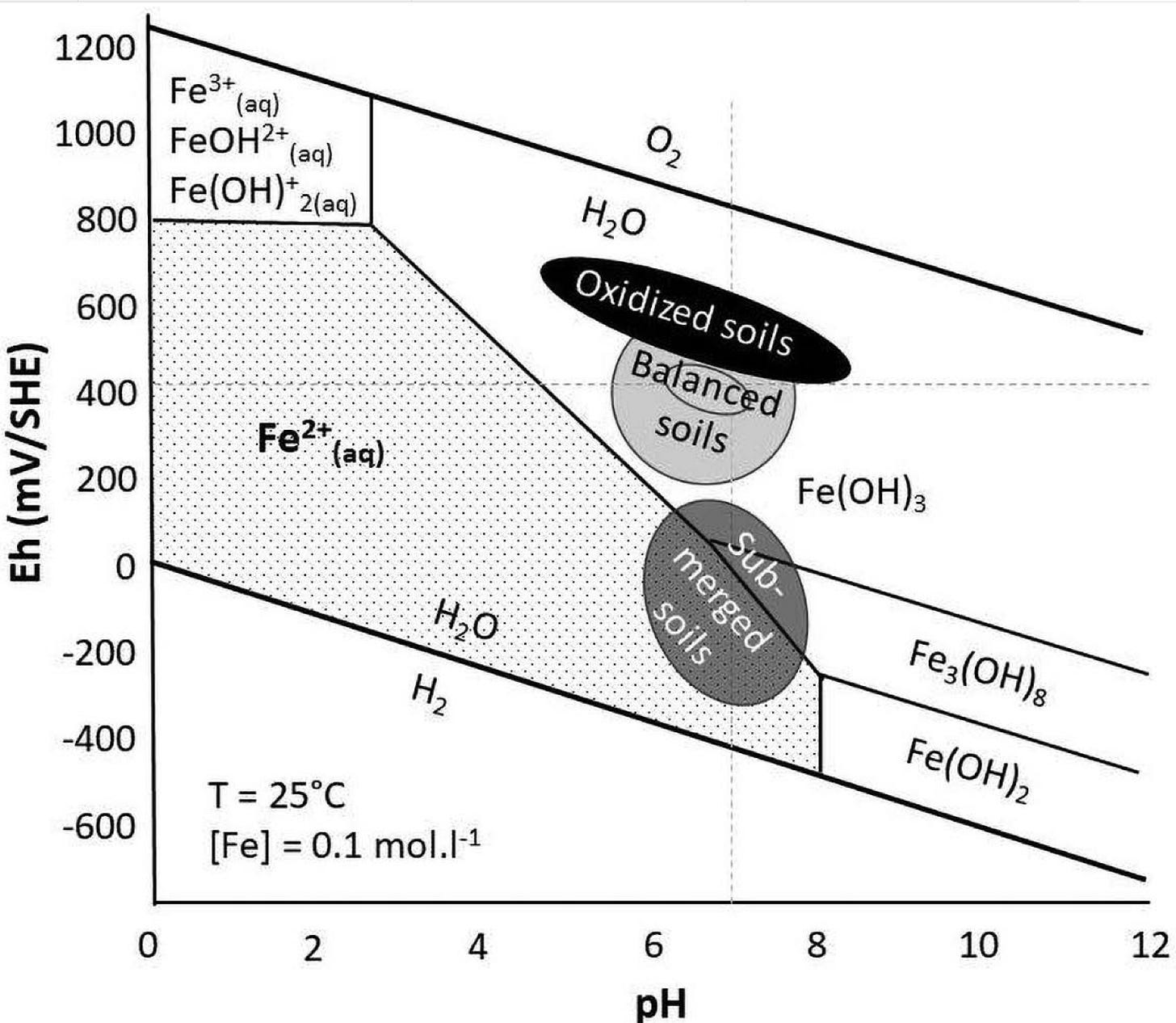
If we look at the pH chart two pictures ago, it says plenty of iron is available at our ideal soil pH of 6.5. But looking at the Pourbaix diagram above we see that at our ideal voltage of 400mV, iron isn’t available at all! Again the form of iron that plants can use and absorb is the soluble or aqueous form FE2+. For a healthy plant in balanced soil, it is not that much of a move to dip down into the usable form. It will take some energy, but not much. However, if we have applied iron in an unusable form, it will take more energy for a plant to get it into the form it needs.
Before you get overwhelmed, with both of these examples above, it’s not about getting Pourbaix diagrams for each and every mineral we want to use. It is highlighting the energetic effect of not using correct forms and the larger impact that has on a plant. All we have to do to minimize the energy expenditure is to simply use the available form to start with. This moves us in the right direction on the voltage-acidity map and does not move us into the insect-pathogen zones. These available forms are readily available in natural fermentations and extracts because the microbiology in them has already broken the minerals down into their aqueous ionic state. Look for soluble or aqueous forms of minerals, not oxidated forms.
Why is nitrogen so difficult?
(Prepare yourself…)
Now let’s take a look at the Pourbaix diagram of nitrogen:
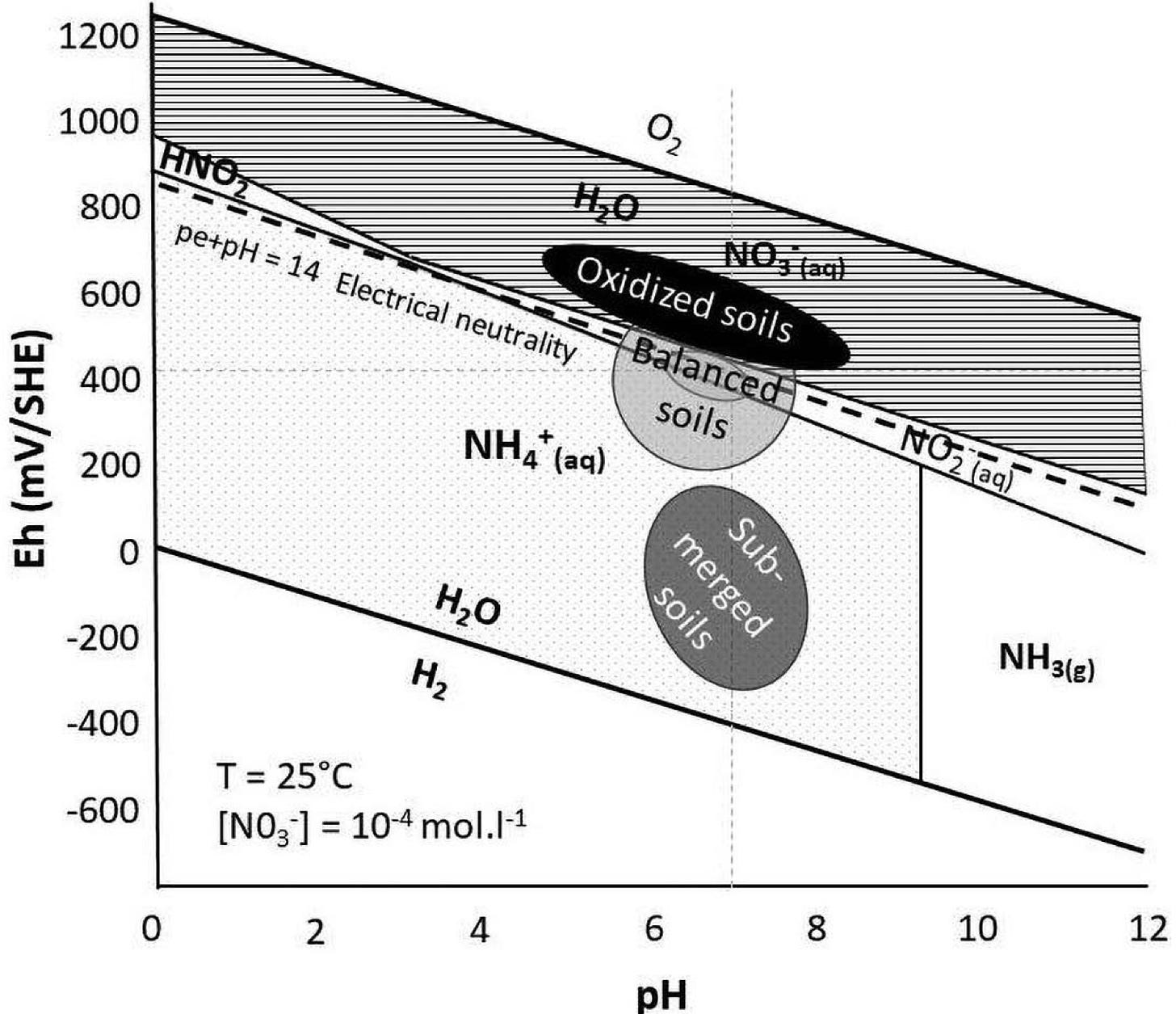
Nitrogen is a very difficult mineral to nail down. It is constantly changing form in the soil. You can see by the diagram above that plants actually use three forms of nitrogen: nitrate (NO3-), nitrite (NO2), and ammonium (NH4+). All three can be available in balanced soil. What affects things is whether our soil is truly balanced and what form of nitrogen we are applying.
A plant absorbing nitrate (NO3-), releases OH-, and a plant absorbing ammonium (NH4) releases H+. This has a huge impact in the soil around the roots because it changes the pH by more than 3 pH units. (Ammonium is below 4.5 pH and nitrate is above 7.5 pH.) If we feed a lot of nitrate, it blocks iron. If we feed a lot of ammonium, it creates toxicity and blocks other nutrients. So we affect our plant’s nutrition and ability to absorb other nutrients just by the form of nitrogen we use. The reason why it is such a big deal is because 80% of the absorbed ions in a plant is nitrogen. If it was, say, just 5% it wouldn’t have as big of an impact on pH and other ions. It is because the plant absorbs a lot of nitrogen that there is a huge impact and huge differences. There is a difference of 8 electrons per nitrogen molecule, so it takes a lot energetic compensation for a plant to make adjustments with it.
Nitrates (NO3-)
Root tips are negatively charged and low voltage. This creates an electric force that attracts positive ions, the cations. This makes for passive absorption of nutrients that doesn’t require much energy. Nitrates also have a negative charge, a repellent charge — think like poles of a magnet— so it takes a lot of work to absorb nitrate because it has to go against the electric gradient. In fact, root respiration increases by 10-15% for this process, that is a lot of energy to transform nitrate just to make it usable.
Nitrate also increases pH, which is a problem if you have alkaline soil. So now you have the energetic cost to change nitrate into ammonium and then to further break it down for amino acids which is greater than 15%, plus the pH correction, plus the 8 electrons per nitrate molecule. So a plant has lost at least 25% of its energy just to work with nitrate.
Nitrate also causes a huge increase in water need for your plant. The reason why is that in order to convert nitrate to ammonium, it takes the “H” that is needed from water (H2O) and then OH- is released back into the environment as a byproduct. You need 4 molecules of water for every 1 molecule of nitrate. That is a tremendous water cost. You have effectively doubled the water requirements for your crop by using nitrate. Because the plant is absorbing so much water, it causes the plant cells to elongate which to us looks like huge growth. But inside, it is full of water and the produce will likewise be full of water and very low on nutrition and flavor.
There is a research paper on nutritional content in amaranth fed on nitrates. The nutritional content was decreased because of it— total flavonoids decreased by 15-17% and total phenolic content reduced by 20-23%. There is a clear correlation in vegetables and other produce between water content and nitrate content. Nitrates are not good for health.
So I hear you, you’re thinking, no problem, I’ll just use ammonium (NH4+ aka urea.)
I’m telling you, nitrogen is a pain in my boo-tay! At least it is to write about it. 😜
Ammonium (NH4+) is an antagonist to the absorption of calcium and magnesium. High ammonium will create deficiencies of these and other nutrients. Ammonium also decreases soil pH, which is bad if you already have acidic soil or if you rely on peat moss in your potting mixes. High amounts of ammonium causes toxicity. It causes low voltage in the soil, and with voltage reduced, the plant’s ability to absorb nitrogen decreases by quite a lot. Ammonium is also not very mobile in the soil, so a plant needs to spend more energy to find it. It has to catch it where it is. So the plant will use energy to send out roots to look for it or will have to outsource to mycorrhizae to find it, spending energy to attract and grow it.
Here is another kicker: You can apply urea nitrogen (ammonium) to your soil, but if your soil is oxidized, a few hours later that ammonium has turned into nitrate because of how volatile it is. It is this fast turnover, even within hours, that makes it hard to study nitrogen and plants. Plus you have competition with microbes. So it’s difficult for researchers to say what a plant is really using.
Has nitrogen given you a headache yet? What do we use for nitrogen then?
If a plant has a choice between nitrate, ammonium, and amino acids, it will take in mainly amino acids. Remember, a plant breaks down the nitrogen it has absorbed for the point of amino acids. Worm castings are good for that, we also have fish amino acids, plus others.
58-68% of the nitrogen a plant uses is from organic matter in the soil, regardless of the fertilizers you put on the ground. (See page 39.) When we are using chemical nitrogen fertilizers, we want to be very aware of when and how we’re using of it, and how it affects the soil structure and stability. Because a lot of it is not being used by the plant, a lot of it is being runoff into the environment. We are talking costs here and lost money.
The nitrogen used by a plant is going to be taken from the most active and stable source of it. That is always going to be from the soil organic matter when it is available. 2% of organic matter contains 2,000 lbs of nitrogen per acre. That is a lot of nitrogen, and as organic matter increases, that amount increases exponentially as well. If we have healthy soil with a lot of organic matter and a steady supply of it on the surface, then it is very likely we do not need to add any nitrogen. If we have nitrogen fixing microbes in the soil, then we do not need to add more on top of their efforts. We haven’t even talked about how plants can also absorb nitrogen from the air through their leaves which is a whole other point. The key is making sure we have enough nitrogen in the soil by means of the biological activity in the soil and through the generation of organic matter. This is what charges the soil battery and keeps things in the healthy energetic spectrum that bugs bounce off of.
Entering homeostasis…
Everything works well. Plants are making photosynthates, they are still pumping a little into the soil to feed the microflora. Most of the energy is used to produce new leaves, so photosynthesis is increasing, giving more energy to the plant. There are balanced amounts of nitrate and ammonium, or even better, we have organic nitrogen which is important to the functioning of the plant. We have low to no toxicities. These plants are not attractive to insects and they do not develop disease. We have huge biomass production, with one part of it mineralized to feed the next plant or season, and the other part used for humis which sustains voltage and acidity at proper levels.
How do we minimize energy outputs and keep things in the no fly zone? Foliar sprays. If we detect any possibility of nutrient deficiencies, or if we just wish to feed our plants, foliar sprays of usable forms of soluble ionic minerals work well to quickly correct imbalances. Soil drenches of usable aqueous minerals are also good for regular feeding. (Aqueous minerals are not chemical fertilizer powders dissolved in water, those are still in oxidized forms. Aqueous minerals are those in their ionic forms that are ready for plant use so no Eh-pH changes occur and no energy is spent to convert them.) Allow organic matter to build up on top of the soil to keep the battery charged and stable. We use the power of knowledge and our power of choice to use inputs that have little effect on voltage and acidity.
It takes plant energy to run the whole system. It’s plant energy that gives us oxygen, it’s plant energy that gives us food, it’s plant energy that gives us topsoil, it’s plant energy that makes the fossil fuels that run our economy. It is all about managing that energy.
What is the bug zapper? It is health. Maybe not quite what you pictured at the onset, but isn’t that the easiest solution? Insects are here all of the time checking out the frequencies to do their nature assigned task of clean-up. We just need to keep the frequencies in the non-detectible range to zap that insect elsewhere. And now with this article, you can. So it’s not just plants that have power, you have become the He-Man of plant electricity and now you’ve got the power!
(Please tell me I’m not the only one hearing He-Man in my head reading about plant electricity! 😂)
And with that, here is the anthem for this article. Sing it to your plants as you adjust their electrical fields. 😏
This article was heavily inspired by Olivier Husson’s work on soil redox, specifically these articles:
Link to Olivier Husson’s other papers: https://www.researchgate.net/profile/Husson-Olivier
And it was also inspired by Nigel Palmer, specifically this article:
Both have many YouTube lectures available and I highly recommend checking them out for more information.









